To address the challenge of refractory, drug-resistant epilepsy, Akumentis Healthcare, a Thane-based pharmaceutical company owned by India’s Akums Drugs and Pharmaceuticals, launched the cannabinoid drug Clasepi last month.
This is a prescription cannabidiol (CBD) product approved by the Drug Controller General of India (DCGI) and is the first cannabidiol medicine of its kind launched in India. Clasepi is characterized by a tetrahydrocannabinol (THC) content of less than 0.1%, giving it non-psychotropic properties. Specifically formulated to treat epileptic seizures associated with the following conditions in patients 1 year of age and older:
-Lennox-Gastaut Syndrome (LGS)
-Dravet syndrome
-Tuberous Sclerosis Complex (TSC)
Clasepi marks the development of India’s first synthetic CBD product. Clinical studies demonstrate Clasepi’s efficacy in reducing seizures, particularly in situations where traditional anti-epileptic drugs have proven ineffective.
It has to be said that Clasepi packaging is somewhat similar to the famous Epidiolex. Epidiolex is an anti-epileptic CBD drug developed by the British pharmaceutical company GW Pharma and has been approved for marketing in the United States and the European Union.
CBD has significant effects on refractory and drug-resistant epilepsy
According to the World Health Organization (WHO), epilepsy accounts for a significant proportion of the global disease burden, affecting approximately 50 million people worldwide, with 20% of these patients residing in India. WHO claims that with the correct use of graduated anti-epileptic drugs, up to 70% of people with epilepsy can become seizure-free.
Epilepsy is a common chronic disease in neurology. Various causes can cause abnormal discharge of neurons, leading to epileptic seizures. Depending on the source of the abnormal discharge, it can have different manifestations. About two-thirds of epilepsy patients’ symptoms can be controlled by anti-epileptic drugs, but there are still some epilepsy patients who respond poorly to drugs. Intractable epilepsy brings severe mental pressure and financial burden to patients and families.
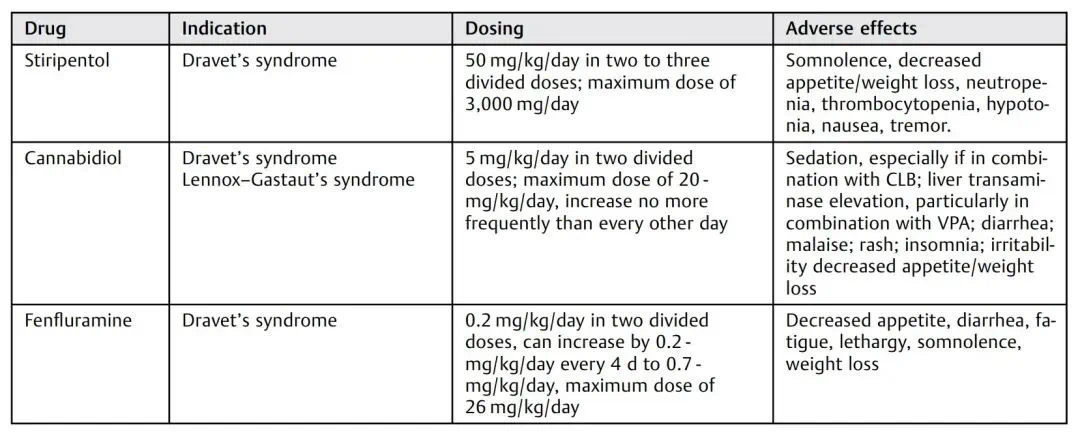
A study (DOI: 10.1055/s-0040-1702941) describes the therapeutic effects of stiripentol, cannabidiol, fenfluramine and other drugs and the ketogenic diet therapy.
The therapeutic mechanism of stiripentol (STP) is mainly through direct action on the neurotransmitter gamma-aminobutyric acid (GABA) receptors, enhancing GABAergic neurotransmission, while inhibiting a variety of hepatic cytochrome P450 enzymes, and increasing some anti-epileptic drugs. blood concentration of the drug, thereby exerting its anti-epileptic effect. Common adverse reactions of STP include drowsiness, agitation, and decreased appetite. The ketogenic diet is a high-fat, low-carb, and moderate-protein diet that has shown promising results in the treatment of drug-resistant epilepsy.
A small prospective trial from China in which 20 children (9 months to 11 years old) diagnosed with Dravet syndrome were put on a 4:1 ratio of the classic ketogenic diet for 6 months found that 17/20 (85%) experienced a reduction in seizure frequency, and more importantly, 17 patients who continued the ketogenic diet for 6 months had no status epilepticus attacks.
Lennox-Gastaut syndrome (LGS) is a severe developmental epileptic encephalopathy that occurs in childhood. It mostly occurs in children aged 2 to 8 years, and is most common in children aged 3 to 5 years. The main characteristics of LGS are multiple seizure types, widespread slow spike-slow complexes (1.5 to 2.5 Hz) in the electroencephalogram, and intellectual disability/developmental delay.

